Mindset XR Module 2: What is a medical device?

Welcome to the Mindset Extended Reality (XR) for digital mental health programme learning resources, which include three series: medical regulation, clinical evidence and lived experience involvement. Mindset-XR is helping to catalyse the growth of immersive digital mental health solutions in the UK, through funding, tailored support and training. It is delivered by Innovate UK and the Health Innovation Network South London (HIN).
This series focuses on medical regulation, with key insights from Hardian Health. Across 10 modules, we provide an accessible introduction to people and companies that want to learn more about medical device regulation, with a focus on XR devices. Each module offers a high level overview of a different topic, including medical device regulation in the UK and EU, core medical device standards and overseas regulation. Each module includes additional resources to support your learning and a quiz to test your understanding.
Outline
What are the UK and EU definitions of a medical device?
Defining medical devices and the differences between UK and EU definitions.
Why is it important to set out intended use?
How the intended use of your medical device will impact its definition and status.
What is Software as a Medical Device (SaMD)?
Understanding when software can be defined as a medical device.
Multiple choice questions to test your understanding on medical device definitions in the UK and EU.
Welcome to Module 2: Am I building a medical device? In this section, we're exploring the definitions and terms that define medical device software classification, as related to XR mental health devices. Topics include:
What are the UK and EU definitions of a medical device?
It’s important to understand the medical device definition of your target market so that you can properly assess whether or not your product is considered a medical device. Each jurisdiction will have a different definition as well as supporting documents to help you assess the definition of their product, such as the MDCG guidance documents for the EU Medical Device Regulation (EU MDR). Below are the current medical device definitions in the UK and EU:
Why is it important to set out intended use?
Ultimately, whether or not your device is a medical device or comes down to the intended use. We will cover more detail on the contents of the intended use statement in module 6. To assess the intended use of your product, you should consider:
Definition
How does your product align with the UK and EU medical device definitions? Do any of the terms or phrases from the official definitions be applied to your product?
Purpose
What purpose does your product fulfil in a medical setting? Some terms that suggest the purpose of your product are: "alleviates", "can benefit those who suffer from" and "clinically proven".
For example, a VR device with associated software that delivers cognitive behavioral therapy (CBT) with the intention to alleviate symptoms of depression is very likely to be considered a medical device under both the UK and EU definition.
In borderline cases, having a clear intended use is vital in understanding whether any of the features of the product would be considered medical device features.
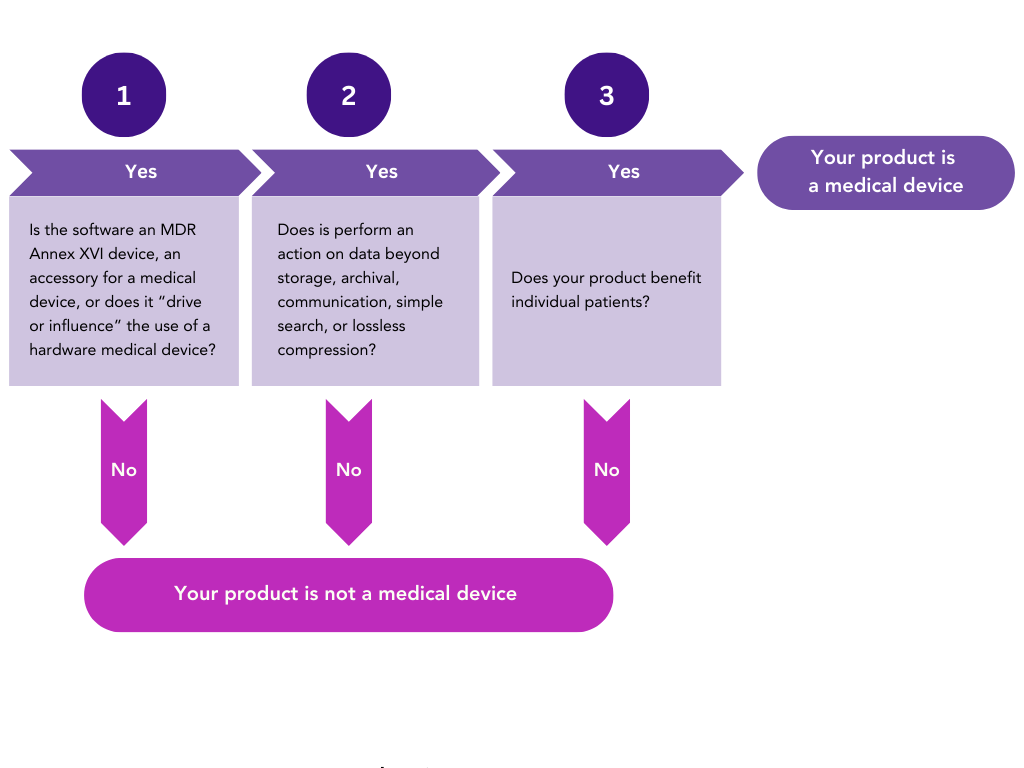
What is Software as a Medical Device (SaMD)?
Now let’s take a close look at software in particular, as many XR medical devices will involve software or various modules as a vital component.
For the purposes of regulation, software is defined as “a set of instructions that processes input data and creates output data”. Terms you will often see used include:
Software as a medical device (or SaMD)
Software that is used as a medical device, without being part of a physical medical device (hardware).
Software in a Medical Device (SiMD)
Software that is part of a physical medical device (hardware).
AI as a medical device (AIaMD)
AI technologies that are used to help medical professionals and patients.
Summary
The exact definition of a medical device varies by jurisdiction, so it is important to understand the regulations applicable to any country you plan to place the device on the market in.
Intended use statement is very important. It helps you understand your medical device status and risk classification, and communicate this with others. It is also one of the most important documents which submitted to the regulatory authorities and can help with communicating the value and limitations of your product to customers and payers.
Be clear and consistent with your claims. Anything you claim has to have evidence to back it up!
In this module, Am I building a medical device?, we explored the definitions of medical devices in the UK and EU, as well as the importance of intended use for understanding your medical device status. After using this resource, you should have a understanding of the following:
Quiz