Mindset XR Module 15: Planning and resources
Welcome to the Mindset Extended Reality (XR) Innovation Support Programme learning resources, which include three series delivered in conjunction with our expert Mindset-XR programme partners:
• Medical regulation
• Clinical evidence
• Lived experience involvement
Mindset-XR is helping to catalyse the growth of immersive digital mental health solutions in the UK, through funding, tailored support and training. It is delivered by Innovate UK and the Health Innovation Network South London (HIN).
This series focuses on research and clinical evidence, with key insights from King's College London's Institute of Psychiatry, Psychology and Neuroscience. Across a number of modules, these resources will guide you through your research journey, from establishing what you plan to investigate, to conducting research and disseminating your findings.
Outline
Key aspects to consider when planning XR research
Understanding the importance of protocols, ethics, and oversight in the planning of XR research.
Resources to account for in your XR research
A list of resources that will be essential for delivering your study.
- Summary
Welcome to Module 15: Planning and resources. In this section, we're focusing on:
Planning
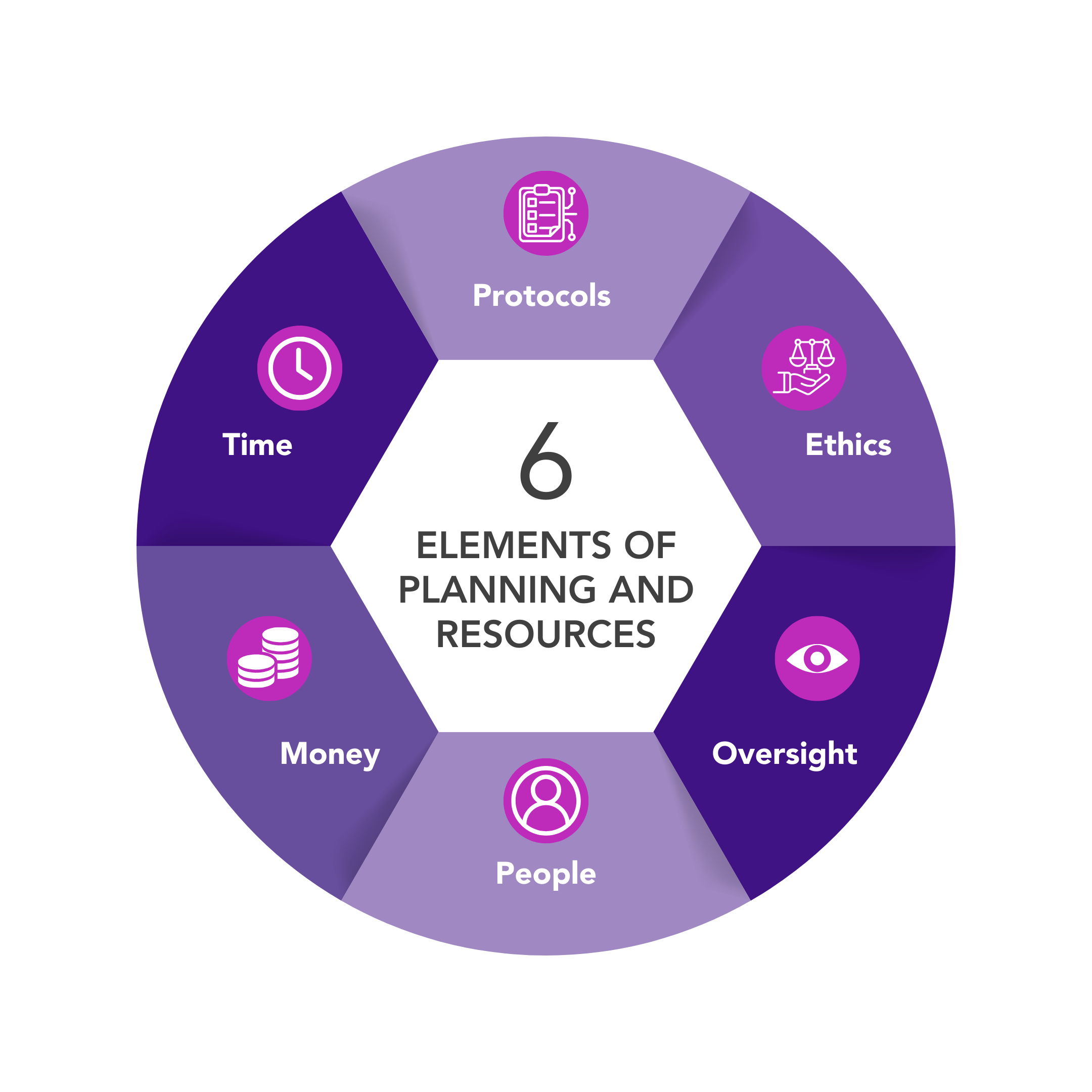
When planning your XR research, these are the important aspects to consider:
Protocols
Protocols hold the key to everything as they play a crucial role in clinical science. They will set out to answer in detail from the very beginning of the project, the "what", "why" and "how" of the research. It will include:
- Background information
- Research questions
- Research methods
- Analytic strategy
Best practice is to pre-register your protocol, often in the form on an open access publication.
This allows the wider community of interest around the research to ensure that you have reported the project in line with the protocol.
Ethics
Part of writing the protocol requires you to be clear on the necessary ethics and governance approvals.
Ethical approvals will need to be in place prior to any data collection.
Do not underestimate the time it takes to gain ethics, particularly in the context of XR research which involves patient groups. Module 16 outlines the ethics processes and gives an idea of the expected time-frames.
It is also important to consider the regulatory requirements to conduct the research. The regulatory landscape in digital health interventions is complex and subject to change over time. Failure to consider this is a threat to your research.
Oversight
Clinical research is typically subject to independent oversight by experts in the field.
In the context of a clinical trial for example, this might involve annual or bi-annual meetings of an independent Data Monitoring and Ethics Committee (DMEC). The purpose of the DMEC is to monitor areas of research integrity, data, and safety.
- Procedures for oversights should be set up early in the lifecycle of the project and continue for its duration.
Resources
In planning of research, it is important to consider the resources you will need. Particularly the people, money, and time it will take to deliver the research.
People
It’s important you have enough capacity in terms of staff. You need to recruit the right blend of skill, knowledge, and experience. Specific areas of expertise to consider include:
- Statistical and health economic methods
- Software development
- Patient and public involvement
- Medical technology regulation
Bear in mind that different disciplines and professions will have different methods and ways of working. You will need to plan how contributions from each team member will combine to deliver your research aim.
Money
Costs linked to paying participants and staff also need to be carefully planned. Some costs for staff that you might not have considered include:
Time
The typical project will involve an initial set up phase, followed by recruitment and data collection. Analysis will then be completed before the research is disseminated.
- Gantt charts are a common and important tool for planning around time-required delivery research tasks.
This chart would normally be split across defined work packages each of which would have associated milestones and outputs. These then feed into the next phase of the research project.
Summary
It is often tempting to try to push research forward quickly. However, by taking the time to carefully plan, you will be in the position to mitigate issues that might impact on the viability of your research. This will in turn maximise the chances of you delivering impactful research.


Next module - Module 16: Research ethics
Back to Module 14: Research design